ABSTRACT:
As the number of positive cases for SARS-CoV2 increases, many of us would like to understand what can be done if we develop symptoms of COVID-19 after exposure, and what are the current best medical practices to preserve our health.
What medical treatments could your doctor offer if your infection progresses?
Although a cure has not been discovered yet, there are some advances in the intervention of the patients that we can implement if a COVID-19 diagnosis is determined. Like any other medical condition, decisions for the management of the viral infection are implemented on a case-by-case basis, and you should consult your physician for the treatment that suits you best.
NIH experts have delivered a series of recommendations with the current evidence. We went over the published scientific articles to explain what all of this means for you. So please, get comfy and put on your nerdy hat because you are going to need it for this one!
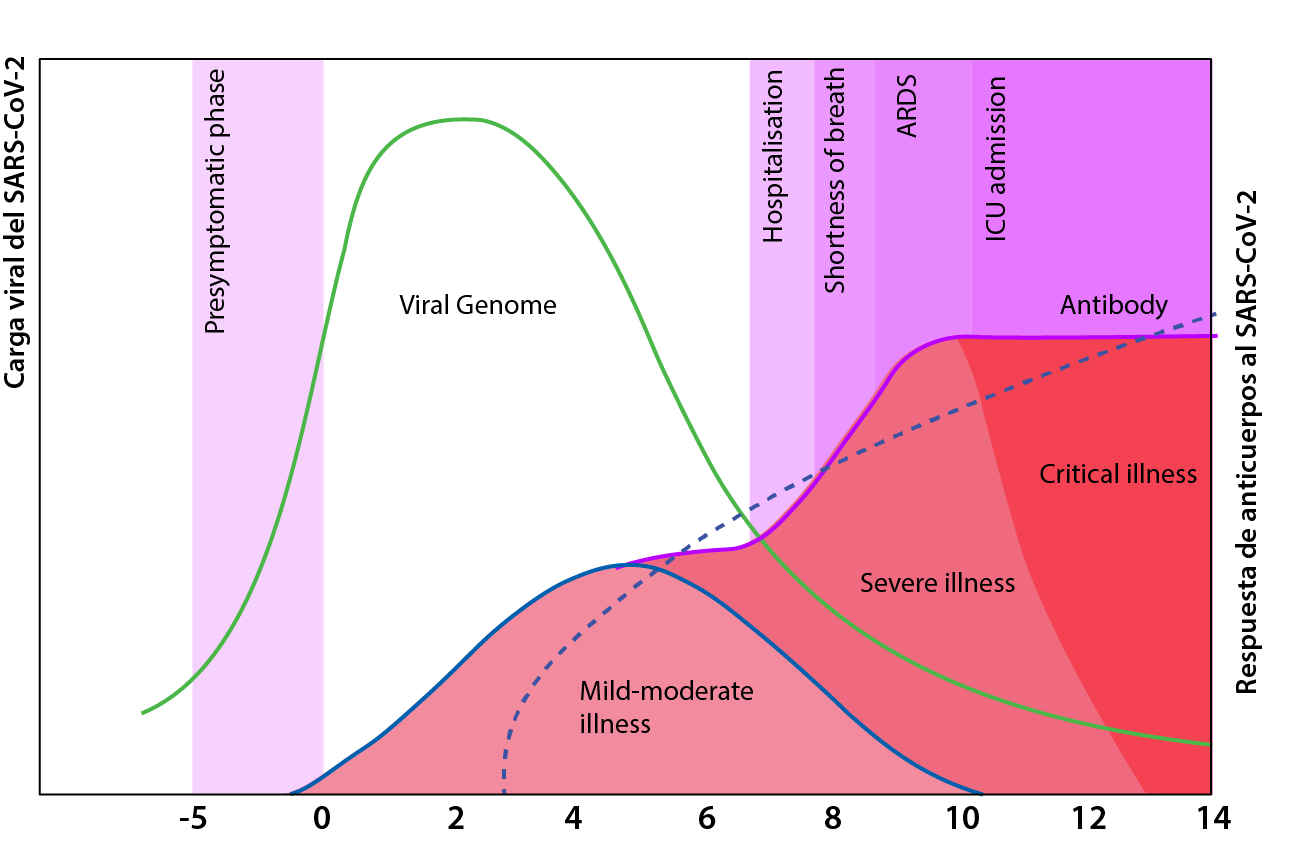
The infection timeline presents an early and late phase. The early phase of the disease is driven by the viral invasion of the respiratory tract, and although it can affect other systems such as the nervous, and gastrointestinal, it’s respiratory system complications that are the main contributor to the rapid deterioration of the majority of patients. In some cases, this is then followed by a severe inflammatory response, which puts the patient’s life on the line and affects their ability to breathe on their own. These different stages explain why some treatment regimens are beneficial at a given point in the disease course but useless or even harmful at other disease stages.
To estimate where you are in the infection, we use the disease severity scale, which is based on how much help you require to breathe.[1]
With this approach, we can divide the patient presentation by
1) no oxygen required
2) low flow oxygen delivery
3) high flow oxygen delivery
4) advanced life support measures.[2]
-There are three main groups of pharmacologic therapies that we will discuss based on recommendations from current evidence:
Immune-based therapies that modulate the hyperactive inflammatory effects from the virus.
Antivirals that directly disrupt part of the viral cycle.
Adjuvant therapies, which are substances that may play a significant role in the treatment or even prevent the infection or its complications.
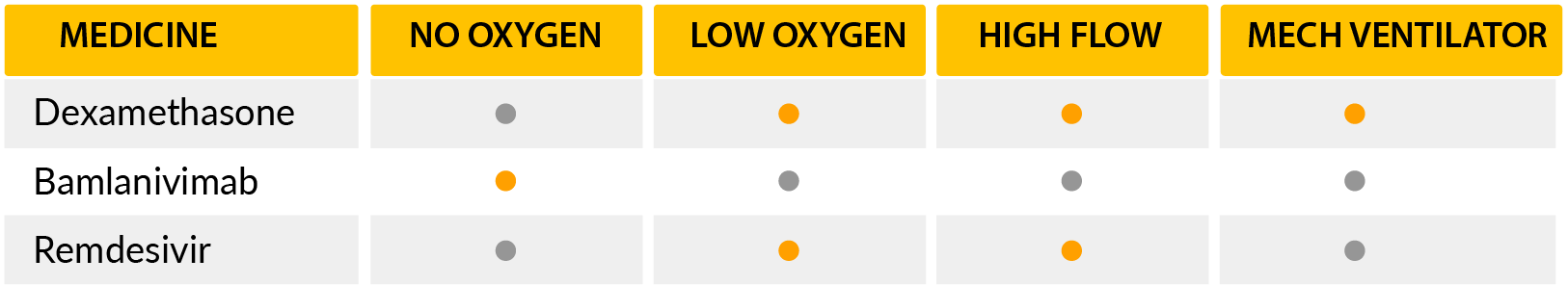
-Several medications have been studied but only one has shown a definitive benefit on patient mortality: Dexamethasone. [9]
Dexamethasone is a corticosteroid drug that is very similar to hormones produced in your body. It works by decreasing the activation of several functions of the immune system, which in turn decreases inflammation. A large study from the UK showed the benefits of this medication on the treatment of COVID19 patients that require oxygen.
This benefit was most evident in patients that required advanced means of life support, such as mechanical ventilation or extracorporeal oxygenation. Researchers’ findings determined a reduction in mortality risk of death of 11.7% and estimated that for every 9 patients one will benefit from this therapy. There was also a minor decrease in mortality from those requiring oxygen delivery by other modalities. In this group of patients, the reduction in the absolute risk of death was 3.5%, estimating that from every 29 patients one will benefit from this therapy. [12] On the other hand, dexamethasone was not beneficial in patients who didn’t require oxygen.NIH recommendation for this medication is that only for use in patients that are hospitalized and require oxygen. Although this is far away from even being considered a cure, it’s the greatest benefit from any medication so far.
Nonetheless, other medications are being tested… like Bamlanivimab. Bamlanivimab is a monoclonal antibody that provides antibodies that neutralize the virus. The clinical trial that justified emergency use authorization by the FDA showed a reduction in hospitalization in 72% of the patients with early diagnosis by PCR. In fact, if Bamlanivimab was infused within 72 hours after a positive result, only 1.6% of the patients went on to require hospitalization compared to 6% of patients that received placebo. A cocktail similar to this medication was in the news in October as it was one of the medications the former president Trump received during his hospital stay.
Another medication is the famous Remdesivir, which works by decreasing viral replication. This makes the medication more beneficial at the early stages of the disease as shown by the adaptive COVID-19 treatment trial. This study concluded that this drug decreased the time to recovery from 15 days on average to 10 days when patients required supplemental oxygen during hospitalization. This benefit was not present when patients had progressed to required advanced life support measures. The WHO contradicts the use of this medication based on a large observational study that showed no benefit of the treatment in hospitalized patients.
Ivermectin on the other hand is a medication that has shown a correlational decrease in reported cases in some populations. The retrospective analysis put this anti-parasite medication as a potential prophylactic alternative for the virus if clinical trials are able to determine that Ivermectin is responsible for this correlation. In laboratory experiments, it decreased viral replication. Therefore, more studies are needed to determine this drug’s potential preventive benefits.
Honorable mentions for COVID-19 treatment methods go to prone positioning. This involves placing mechanically ventilated patients face-down, allowing for better lung function. Despite its physical limitations, this technique has also shown some potential benefit even before the patient requires ventilation.
Amongst the adjuvant therapies are vitamin C, vitamin D, and Zinc. Although they lack strong randomized clinical trials for the treatment of COVID-19, their use has been proven to be safe for most patients. The control of chronic medical conditions such as diabetes and hypertension decreases the risk for complications from the infection as well.
CONCLUSION
In conclusion, Bamlanivimab could prevent progression to hospitalization when given early in the disease. Remdesivir may decrease the duration of hospitalizations of patients requiring oxygen who are not intubated. Dexamethasone can be used to decrease mortality for patients who receive oxygen, especially those who are on mechanical ventilation. Given the limited treatment options, patients at risk should maintain good control of their chronic conditions.
As vaccines become available, the consequences of the infection are dependent upon the prevention of the disease spread. Your role is critical to protect your loved ones as much as yourself. This is the importance of physical distancing, hand hygiene, and mask-wearing.
REFERENCES:
- Beigel, J. H. et al. (2020) ‘Remdesivir for the Treatment of Covid-19 — Final Report’, New England Journal of Medicine. Massachusetts Medical Society, 383(19), pp. 1813–1826. doi: 10.1056/nejmoa2007764.
- Alhazzani W, Møller MH, Arabi YM, Loeb M, Gong MN, Fan E, Oczkowski S, Levy MM, Derde L, Dzierba A, Du B, Aboodi M, Wunsch H, Cecconi M, Koh Y, Chertow DS, Maitland K, Alshamsi F, Belley-Cote E, Greco M, Laundy M, Morgan JS, Kesecioglu J, McGeer A, Mermel L, Mammen MJ, Alexander PE, Arrington A, Centofanti JE, Citerio G, Baw B, Memish ZA, Hammond N, Hayden FG, Evans L, Rhodes A. Surviving Sepsis Campaign: Guidelines on the Management of Critically Ill Adults with Coronavirus Disease 2019 (COVID-19). Crit Care Med. 2020.
- Guan WJ, Ni ZY, Hu Y, Liang WH, Ou CQ, He JX, Liu L, Shan H, Lei CL, Hui DSC, Du B, Li LJ, Zeng G, Yuen KY, Chen RC, Tang CL, Wang T, Chen PY, Xiang J, Li SY, Wang JL, Liang ZJ, Peng YX, Wei L, Liu Y, Hu YH, Peng P, Wang JM, Liu JY, Chen Z, Li G, Zheng ZJ, Qiu SQ, Luo J, Ye CJ, Zhu SY, Zhong NS; China Medical Treatment Expert Group for Covid-19. Clinical Characteristics of Coronavirus Disease 2019 in China. N Engl J Med. 2020 Apr 30;382(18):1708-1720. doi: 10.1056/NEJMoa2002032. Epub 2020 Feb 28. PMID: 32109013; PMCID: PMC7092819.
- Zhao H, Wang H, Sun F, Lyu S, An Y. High-flow nasal cannula oxygen therapy is superior to conventional oxygen therapy but not to noninvasive mechanical ventilation on intubation rate: a systematic review and meta-analysis. Crit Care. 2017 Jul 12;21(1):184. doi: 10.1186/s13054-017-1760-8. PMID: 28701227; PMCID: PMC5508784.
- Xu XP, Zhang XC, Hu SL, Xu JY, Xie JF, Liu SQ, Liu L, Huang YZ, Guo FM, Yang Y, Qiu HB. Noninvasive Ventilation in Acute Hypoxemic Nonhypercapnic Respiratory Failure: A Systematic Review and Meta-Analysis. Crit Care Med. 2017.
- Tsolaki V, Siempos I, Magira E, Kokkoris S, Zakynthinos GE, Zakynthinos S. PEEP levels in COVID-19 pneumonia. Crit Care. 2020 Jun 6;24(1):303. doi: 10.1186/s13054-020-03049-4. PMID: 32505186; PMCID: PMC7275848.
- Munshi L, Walkey A, Goligher E, Pham T, Uleryk EM, Fan E. Venovenous extracorporeal membrane oxygenation for acute respiratory distress syndrome: a systematic review and meta-analysis. Lancet Respir Med. 2019 Feb;7(2):163-172. doi: 10.1016/S2213-2600(18)30452-1. Epub 2019 Jan 11. PMID: 30642776.
- Bos LDJ, Paulus F, Vlaar APJ, Beenen LFM, Schultz MJ. Subphenotyping Acute Respiratory Distress Syndrome in Patients with COVID-19: Consequences for Ventilator Management. Ann Am Thorac Soc. 2020 Sep;17(9):1161-1163. doi: 10.1513/AnnalsATS.202004-376RL. PMID: 32396457; PMCID: PMC7462326.
- Horby, P. et al. (2020) ‘Hydroxychloroquine for COVID-19-Preliminary Report Effect of Hydroxychloroquine in Hospitalized Patients’, medRxiv. Cold Spring Harbor Laboratory Press, p. 2020.07.15.20151852. doi: 10.1101/2020.07.15.20151852.
- Cavalcanti AB, Zampieri FG, Rosa RG, Azevedo LCP, Veiga VC, Avezum A, Damiani LP, Marcadenti A, Kawano-Dourado L, Lisboa T, Junqueira DLM, de Barros E Silva PGM, Tramujas L, Abreu-Silva EO, Laranjeira LN, Soares AT, Echenique LS, Pereira AJ, Freitas FGR, Gebara OCE, Dantas VCS, Furtado RHM, Milan EP, Golin NA, Cardoso FF, Maia IS, Hoffmann Filho CR, Kormann APM, Amazonas RB, Bocchi de Oliveira MF, Serpa-Neto A, Falavigna M, Lopes RD, Machado FR, Berwanger O; Coalition Covid-19 Brazil I Investigators. Hydroxychloroquine with or without Azithromycin in Mild-to-Moderate Covid-19. N Engl J Med. 2020 Nov 19;383(21):2041-2052. doi: 10.1056/NEJMoa2019014. Epub 2020 Jul 23. Erratum in: N Engl J Med. 2020 Nov 19;383(21):e119. PMID: 32706953; PMCID: PMC7397242.
- Song, Z. fang et al. (2003) ‘Evaluation of glucocorticoid in treatment for patients with acute respiratory distress syndrome’, Zhongguo wei zhong bing ji jiu yi xue = Chinese critical care medicine = Zhongguo weizhongbing jijiuyixue, 15(6), pp. 349–353.
- Drug and Therapeutics Bulletin (2020) ‘Dexamethasone for COVID-19: preliminary findings’. doi: 10.1136/dtb.2020.000045.
- Horby, P. et al. (2020) ‘Dexamethasone in Hospitalized Patients with Covid-19 — Preliminary Report’, The New England Journal of Medicine. doi: 10.1056/NEJMoa2021436.
- Chen, P., Nirula, A., Heller, B., Gottlieb, R. L., Boscia, J., Morris, J., Huhn, G., Cardona, J., Mocherla, B., Stosor, V., Shawa, I., Adams, A. C., Van Naarden, J., Custer, K. L., Shen, L., Durante, M., Oakley, G., Schade, A. E., Sabo, J., Patel, D. R., … BLAZE-1 Investigators (2020). SARS-CoV-2 Neutralizing Antibody LY-CoV555 in Outpatients with Covid-19. The New England journal of medicine, NEJMoa2029849. Advance online publication. https://doi.org/10.1056/NEJMoa2029849
- Meyers, C. et al. (2020) ‘Lowering the transmission and spread of human coronavirus’, 2(September). doi: 10.1002/jmv.26514.

Health Facts Now has the privilege of having Dr. Jhonatan Rivera-Mora as its Director of the Medical Department. He was born in Quito, Ecuador into a Christian missionary family. He completed his doctorate in Medicine at the San Juan Bautista School of Medicine in Caguas, Puerto Rico, in June 2019. In July 2019, Dr. Rivera-Mora began working for Brigham and Women’s Hospital in Boston, Massachusetts as a postdoctoral researcher in the division of Endocrinology, Hypertension, and Diabetes. In this position, he also holds an appointment as a postdoctoral researcher for Harvard Medical School under the auspices of the National Institute of Health (NIH), Dr. Rivera-Mora participates in research activities for the management of diseases such as hypertension. In this scenario, Dr. Rivera-Mora shares time and knowledge with some of the most recognized scientists and doctors in the world in their respective fields.

Thanks for your blog, nice to read. Do not stop.